Send this article to a friend:
July
09
2022

Send this article to a friend: July |
 |
Methanol as fuel: An accessible early step toward clean shipping
The global shipping industry drastically needs to clean up its game, but dragging these massive machines through the ocean requires extraordinary amounts of energy. Cheap, plentiful, filthy marine diesel will be extremely hard to replace. Last year we examined a promising high-density clean fuel candidate in green ammonia, but now we're learning that the industry is starting to potentially tilt toward methanol as the solution instead. So let's take a look at why, and examine methanol's potential as a clean fuel. Why not green ammonia? The answer appears to be fairly simple, according to a report from Recharge News. Ammonia is viewed as unsafe, it's difficult to handle and it simply doesn't carry enough energy. In terms of safety, ammonia becomes harmful to humans at a very low concentration of 30 parts per million (ppm) over long-term exposure, or 300 ppm if exposure lasts an hour. So small leaks in any part of a ship's system could pose a persistent risk to the crew, and any larger release of fuel – say, if the ship runs aground, or hits another ship and the hull's breached, or there's a significant leak during refueling, or somebody drops a shipping container at port and damages part of the fuel system – could easily result in numerous fatalities. Together In Safety's Future Fuels Report lists no less than six risk assessment scenarios in which ammonia fuels could pose an "intolerable risk." Handling is difficult, says the same report, since ammonia only remains liquid under low to medium pressure, or when it's kept below -34 °C (-29 °F), and the shipping industry doesn't currently have regulations, rules or guidelines in place for its use as a fuel – mind you, it's most certainly already being carried in bulk as a cargo. And then there's its volumetric energy density, which is key to viability in the shipping racket. At 3.5 kWh/l, ammonia is considerably more attractive than gaseous or cryogenic liquid hydrogen in this regard, but you get nearly 24 percent more energy per liter with methanol, and it's both easier and safer to deal with. 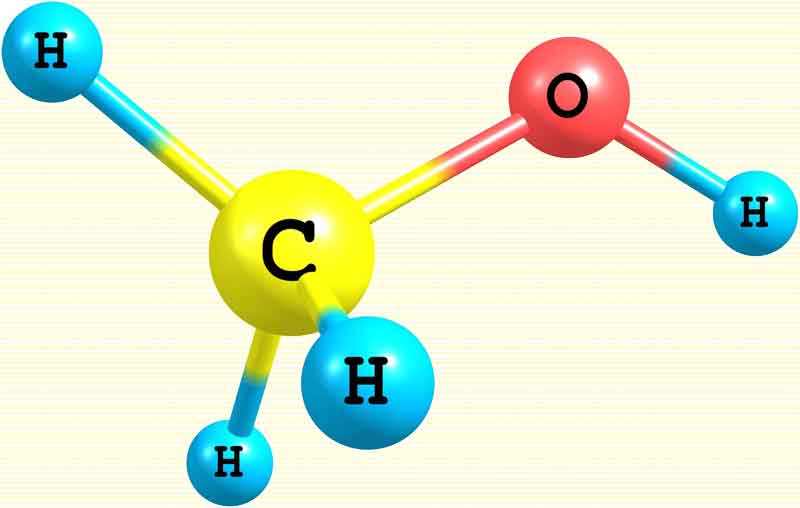 Methanol is the simplest alcohol, and is a light, volatile, colorless liquid with a distinctive odor very similar to that of ethanol Methanol as a fuel today Methanol – chemical formula CH3OH – remains a liquid at ambient temperatures and pressures. It's an established fuel already, with established regulations for its use. It can be used as a combustion fuel in most modern gasoline engines with nothing but a few software tweaks to the fuel injection, and maybe some replacement seals and fuel lines. More extensive adaptations are required to burn it in diesel engines. Its high octane rating has made it popular as a high-power fuel for race cars, monster trucks and dragsters. Roland Gumpert has even launched the Natalie, a methanol fuel cell-powered supercar. While its gravimetric and volumetric energy densities of 5.5 kWh/kg and 4.3 kWh/l are less than half of what diesel can offer (12.7 kWh/kg, 10.7 kWh/l), methanol stands considerably ahead of other clean fuel alternatives on both measures. Methanol is highly flammable – but less so than gasoline; it's harder to ignite and slower to burn, producing just one-eighth of the heat. On the other hand, it burns without a visible flame or smoke, which can make it much harder to detect fires and work out where they're spreading. Water can be ineffective at extinguishing methanol fires, but dry chemical, foam, sand or carbon dioxide extinguishers will do the job. It's toxic to humans if swallowed, or inhaled, or if it contacts the skin, but at least it's a liquid and not an airborne gas, so it scores "broadly acceptable" or "tolerable risk" in all categories in Together in Safety's Future Fuels Report. It requires extra precautions during storage and handling, and needs to be kept in well-sealed tanks, since it's hygroscopic and will absorb water vapor out of the air, diluting its energy content. Having said that, it would fit very easily into existing fueling infrastructure compared to other clean fuel alternatives. It's not entirely accurate to call it a clean fuel though, whichever way you dice it. The carbon atom at the center of the methanol molecule always seems to find a pair of oxygens. Methanol in combustion engines: emissions At high temperatures, two molecules of methanol (2CH3OH) will combine with three molecules of oxygen (3O2) to produce four molecules of water (4H2O) and two of carbon dioxide (2CO2). Yes, carbon dioxide. But even in a combustion setting, methanol burns much cleaner than fossil fuels. According to the Methanol Institute, "compared to conventional fuels, renewable methanol cuts carbon dioxide emissions by up to 95 percent, reduces nitrous oxide emissions by up to 80 percent, and completely eliminates sulfur oxide and particulate matter emissions." So it's definitely a positive step, even if it's not a perfect one. Depending on the availability of methanol, it's possible to design engines capable of running methanol or conventional fuels, or indeed blends of the two. So the barrier to entry here is pretty low, and indeed, according to Recharge News, there are already some 54 methanol or dual-fuel vessels in service or under construction. Most are dual-fuel, because it's currently very difficult to get hold of methanol at cargo-ship quantities, and the fueling infrastructure's not there yet. Going straight to electricity: methanol fuel cells As with hydrogen and ammonia, it's possible to power electric drivetrains and propulsion systems using methanol in a fuel cell arrangement. But again, the carbon atom in each methanol molecule finds itself a pair of oxygens. In a direct methanol fuel cell, or DMFC, the chemical equation works out the same as if you burn it, two molecules of methanol and three of oxygen ending up producing four water molecules and two of carbon dioxide. At this stage, direct methanol fuel cells convert the fuel's energy content to electricity at an efficiency of around 30-40 percent – but in theory, it's possible to get this up to 97 percent, and there's plenty of research going on in this area. 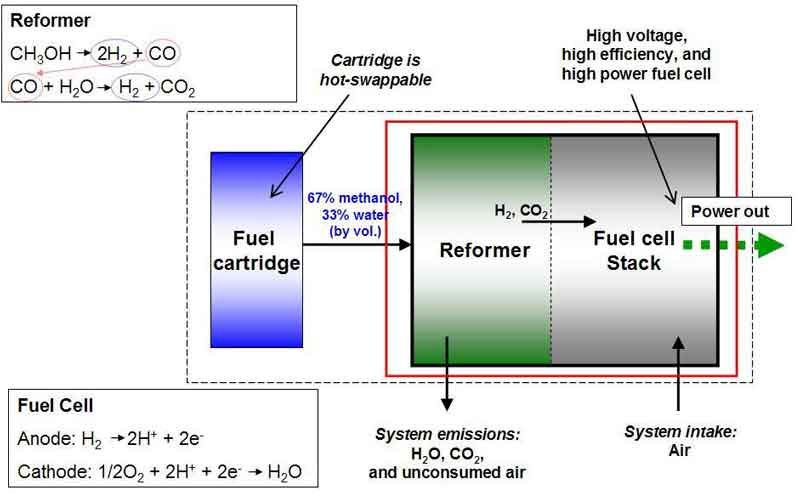
Reformed methanol fuel cell diagram Then there are reformed methanol fuel cells, or RMFCs, which use a steam reformer to convert methanol into hydrogen, and then run the hydrogen directly through a fuel cell stack. These are currently smaller, and capable of reaching efficiencies of 45 percent. They're also effective with lower purity methanol – indeed, some setups are designed for mixtures with up to 40 percent water, although this clearly impacts the volumetric energy density. In an example of a RMFC, the steam reformer might break methanol into two hydrogen molecules and one carbon monoxide, and then that carbon monoxide would combine with a water molecule to produce another hydrogen molecule and a carbon dioxide, leaving you with a total of three hydrogen molecules to run through a standard PEM fuel cell stack, and roughly the same amount of carbon dioxide you'd get from the other two approaches. Methanol production overview You can make methanol in many ways, from the old-school distillation of wood alcohol to renewable methods that involve the recycling of carbon dioxide. Most of it, however, is currently made using natural gas (methane), or coal. In the most common process, natural gas is steam-reformed into a syngas, hydrogen is separated out for separate use, and the remaining methanol/water mixture is distilled to get you pure methanol. This is of course a dirty process; the steam reforming process results in both carbon dioxide and carbon monoxide by-products. There are some other promising methods, including the use of metal-organic framework catalysis to convert methane and sunlight directly into methanol at room temperature – but any process that involves methane gas must be held responsible for the unavoidable fugitive emissions of this extremely potent greenhouse gas that escape from the well, the transmission pipes and fittings.  Enerkem's Edmonton plant diverts municipal garbage and turns it into methanol, ethanol, and other chemical products
Greener methanol production It's possible to create "biomethanol" by gasifying "municipal solid waste," or regular ol' garbage including waste wood, plastics and styrofoams, then running it through a reactor and distilling it. Indeed, Enerkem has had this kind of operation running at a commercial scale for several years now in Edmonton, Canada, diverting as much as 30 percent of the city's waste out of landfills. It's also possible to make methanol using green energy. You'd use the electricity to electrolyze water and separate out the hydrogen, then combine the hydrogen in a reactor with carbon dioxide, presumably captured from another process, and then you'd distil the result to produce e-methanol. However, you're losing energy at each step of this operation. Carbon Recycling International is an example of a company that's been doing this at commercial scale since 2012. Renewable e-methanol and biomethanol projects are popping up here and there, with Europe leading the way. 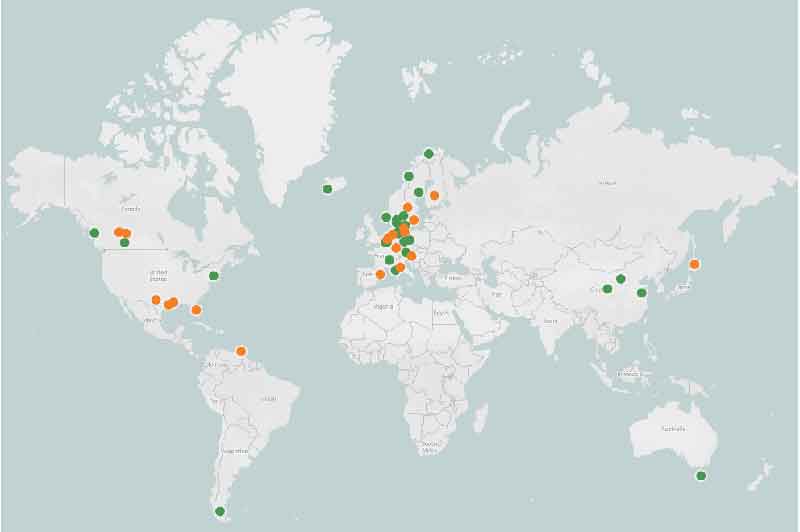
The Methanol Institute's map of global biomethanol (orange) and e-methanol (green) projects What's the overview from a clean shipping perspective? Methanol would appear to represent a relatively accessible way for shipping companies to significantly reduce emissions in the short and medium term. It's not a totally green fuel, but it's probably fair to view it as a greenish-blue in the spectrum of available options, provided it's produced in a clean process. It requires minimal changes to existing combustion engine designs, and offers a better safety profile than ammonia, but it's currently not available at most fueling depots. For a given fuel tank size, it carries less than half the energy of diesel, and this could restrict its suitability to medium-range operations, or necessitate additional fuel stops. It's price-competitive with gasoline, particularly at reduced purity levels, and even at purity levels as low as 90 percent, it'll do the job in a combustion engine. Mind you, it's price-competitive when produced using natural gas or coal – green methanol is more expensive and far less common. In fuel cell-electric implementations, you don't gain much in terms of overall carbon emissions, but if the efficiency of the fuel cells can be improved, methanol-electric ships may eventually be able to travel farther than their methanol-combustion counterparts on a given amount of fuel. Overall, this looks to us like a worthwhile transitional step toward clean shipping, with a fairly low barrier to entry and an opportunity to make a significant early contribution to decarbonization. But if we're to reach zero carbon emissions by 2050, methanol's not going to take us all the way – so despite its issues, ammonia may still be the better solution in the long run, or another candidate may surface. Sources: IEA/Together in Safety/Methanol Institute via Recharge News
|
Send this article to a friend:
 |
 |
 |